Therapeutics Evidence
Based Recommendations
Keeping up with Regulatory Standards and Evidence in Todays Growing Cancer Genomics Industry
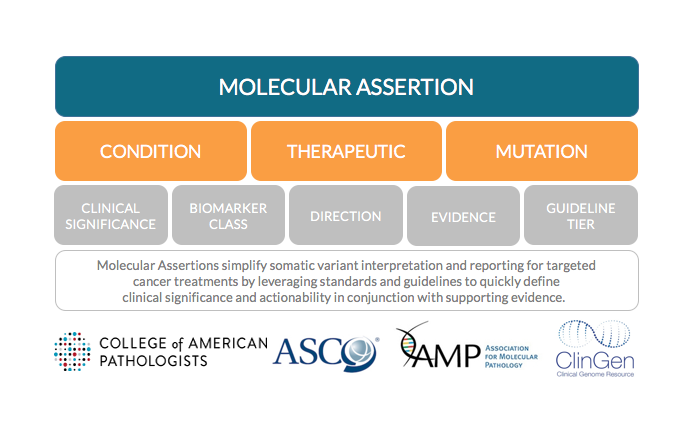
New standards and guidelines published by AMP, CAP and ASCO validate the growing need for somatic variant reporting that comforms to clinical data standards by including scientific rationale that supports treatment recommendations. To optimize and expedite the enduring task of reevaluating clinical significance, MolecularMatch created Molecular Assertions which automate scientific rationale tiering using predefined or custom templates.
- Optimize and standardize your clinical reporting with validated assertions.
- Reduce time and effort associated with frequent reevaluation of publications and determining their level of evidence.
- Pathologists and tumor boards quickly and consistently make evidence based molecularly targeted decisions.
- Gain confidence by complying with standards and guidelines that suit your Lab.
- Easily apply new standard data changes for defining levels of evidence.
- Apply scientific rationale to assay panel development.
MolecularMatch Molecular Assertions > 5,000 and growing
Molecular Assertions establish concrete linkage between a given condition, molecular alteration, clinical significance (prognostic, diagnostic, predictive), therapeutic, and source citation. Decoupled tiering templates draw from evidence attributes listed above such as prospective or retrospective studies, clinical trial phase etc. MolecularMatch collaborates with trusted academic institutions while developing proprietary assertions based on scientific literature. Molecular Assertions improves efficiencies and maintains consistency in how you create reports, design assays and comply with industry standards and guidelines.
Customer Use Case
The assay design team at a diagnostics company was tasked with targeting all of the genomic variations that might influence a clinician’s decision to treat a specific type of cancer. However, the desired size of the panel was too small to survey all of the variations. The customer needed to prioritize the variations by regulatory approval.
MolecularMatch was their solution of choice because we regularly monitor the precision medicine landscape for new evidence supporting the use of therapeutics in cancer. The team utilized the knowledgebase containing rules establishing the clinical relevance of thousands of specific variant-condition-drug scenarios; each backed by specific reference(s) to the literature. The information was dynamically tiered to a custom levels of evidence template based on the customer’s preference (AMP/CAP) so that they could focus on building and validating their design.
Request a Demo of Lab and let us show you efficiencies in your curation workflow
